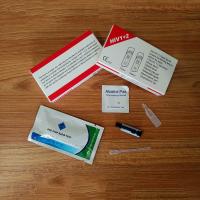
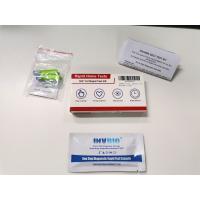
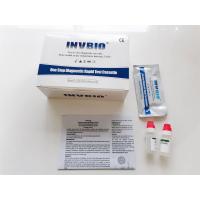
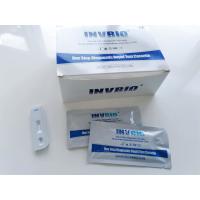
Product Description
Some people are at particularly high risk of becoming infected with
HIV and may be advised to have regular tests.
When to get tested
Seek medical advice immediately if you think there's a chance you
could have HIV. The earlier it's diagnosed, the earlier you can
start treatment and avoid becoming seriously ill.
Some HIV tests may need to be repeated 1-3 months after exposure to
HIV infection, but you should not wait this long to seek help.
Anti-HIV medicine called post-exposure prophylaxis (PEP) may stop
you becoming infected if taken within 72 hours of being exposed to
the virus.
blood test – where a sample of blood is taken in a clinic and sent
for testing in a laboratory. Results are usually available on the
same day or within a few days.
| Brand | Accuracy | Sensitivity | Specificity | Specimen | Packaging |
| INVBIO | 99% | 99.5% | 99.5% | serum/plasma/whole blood | 25 tests/box |
Place of origin: China
Certificate: ISO 13485
OEM packaging is available
Company Information
Innovation Biotech (Beijing) Co., Ltd . is a biotechnology company specializing in research, development
and manufacturing of advanced medical in-vitro diagnostic (IVD)
rapid test kits, medical and laboratory disposal products. We
provide one step medical diagnostic rapid test kit based on our
INVBIO brand, Product include Fertility Tests, Tumor markers, DOA
drug of abuse test, Drug test cup, Urinalysis reagent strip, ELISA
kit, Digital alcohol tester, urine analyzer, et.
OEM packaging is available, drug of abuse test, troponin I test,
alcohol screening saliva test strips, urine strips, elisa kits,
microscope slides
Our focus is to expand our markets internationally by forming
strategic alliances and entering into partnerships with
distributors worldwide. We have established an international
reputation for excellence in the manufacturing of quality medical
and laboratory products. In addition, we have obtained approval
licenses ISO13485, FSC certificate, and most of our products get CE
mark Our objective is the utmost satisfaction of our clients all
around the world by supplying our quality and economical product.
OEM Service

ExpoMedical exhibition in Argentina
Date: from September 26, 2018 to September 28, 2018
Add.: Centro Costa Salguero, Buenos Aires, Argentina
Our booth No: C12 in Hall 4
2022 MEDICA in Germany
Date: from November 14th to November 17th
Add.: Messe Düsseldorf
Hall: 01
Out booth No: 1H39
2023 MEDLAB in UAE
Date: from February 6th to February 9th
Add.: Dubai
Out booth No: Z2.J39

Contact information
Mob/WhatsApp/WeChat/Skype: 0086 13356149018
Email: sales1@rapid-test-kit.com
Website: www.rapid-test-kit.com
FAQ
Q: What is your payment terms?
A: Payment terms: 100% TT before shipment
Q: Can you send samples?
A: Yes,SAMPLES can be sent for your evaluation.customer pay the
freight charge
Q: Do you give any discount?
A: I'll surely try my best to help you get those by the best price
and good service at the same time. We promise the best price upon
same quality, and best quality upon same price.
Q: How to order?
A: Please tell us the model and quantity and package requests.
B. Proforma Invoice confirmed, the order will be arranged upon
receipt of your payment.
C. Confirm and ship the goods.
D. We will help to track your goods until you receive them safely.